Saltmarsh Sparrow Ammospiza caudacuta Scientific name definitions
- EN Endangered
- Names (20)
- Subspecies (2)
Jon S. Greenlaw, Chris S. Elphick, William Post, and James D. Rising
Version: 1.0 — Published March 4, 2020
Text last updated October 4, 2018
Text last updated October 4, 2018
Sign in to see your badges
Species names in all available languages
| Language | Common name |
|---|---|
| Catalan | sit pardalenc d'aiguamoll |
| Dutch | Spitsstaartgors |
| English | Saltmarsh Sparrow |
| English (United States) | Saltmarsh Sparrow |
| French | Bruant à queue aiguë |
| French (France) | Bruant à queue aiguë |
| German | Spitzschwanzammer |
| Icelandic | Merskitittlingur |
| Japanese | トゲオヒメドリ |
| Norwegian | spisshalespurv |
| Polish | bagiennik ostrosterny |
| Russian | Острохвостая овсянка-барсучок |
| Serbian | Strnad iz slanih močvara |
| Slovak | strnádlik pobrežný |
| Spanish | Chingolo Colifino |
| Spanish (Mexico) | Gorrión Cola Aguda |
| Spanish (Spain) | Chingolo colifino |
| Swedish | spetsstjärtad sparv |
| Turkish | Tuzcul Bataklık Serçesi |
| Ukrainian | Багновець гострохвостий |
Ammospiza caudacuta (Gmelin, 1788)
PROTONYM:
Oriolus caudacutus
Gmelin, 1788. Systema Naturae. Editio decima tertia, aucta, reformata. Cura Jo. Frid. Gmelin (etc.). Tomus I [pars I], p.394.
TYPE LOCALITY:
New York.
SOURCE:
Avibase, 2023
Definitions
- AMMOSPIZA
- caudacuta / caudacutus
The Key to Scientific Names
Legend Overview
UPPERCASE: current genus
Uppercase first letter: generic synonym
● and ● See: generic homonyms
lowercase: species and subspecies
●: early names, variants, misspellings
‡: extinct
†: type species
Gr.: ancient Greek
L.: Latin
<: derived from
syn: synonym of
/: separates historical and modern geographic names
ex: based on
TL: type locality
OD: original diagnosis (genus) or original description (species)
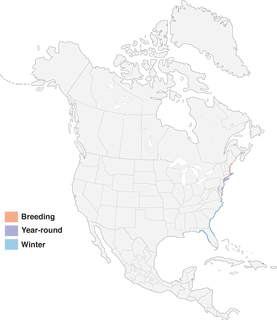
- Year-round
- Migration
- Breeding
- Non-Breeding
Figure 1. Distribution of Saltmarsh Sparrow.






























