Peregrine Falcon Falco peregrinus Scientific name definitions
- LC Least Concern
- Names (68)
- Subspecies (19)
Clayton M. White, Nancy J. Clum, Tom J. Cade, and W. Grainger Hunt
Version: 1.0 — Published March 4, 2020
Sign in to see your badges
Species names in all available languages
| Language | Common name |
|---|---|
| Afrikaans | Swerfvalk |
| Albanian | Krahëthata |
| Arabic | شاهين |
| Armenian | Սապսան |
| Asturian | Falcñn comñn |
| Azerbaijani | Şahin |
| Basque | Belatz handia |
| Bulgarian | Сокол скитник |
| Catalan | falcó pelegrí |
| Chinese | 遊隼 |
| Chinese (SIM) | 游隼 |
| Croatian | sivi sokol |
| Czech | sokol stěhovavý |
| Danish | Vandrefalk |
| Dutch | Slechtvalk |
| English | Peregrine Falcon |
| English (United States) | Peregrine Falcon |
| Faroese | Ferðafalkur |
| Finnish | muuttohaukka |
| French | Faucon pèlerin |
| French (France) | Faucon pèlerin |
| Galician | Falcón peregrino |
| German | Wanderfalke |
| Greek | Πετρίτης |
| Haitian Creole (Haiti) | Grigri pelren |
| Hebrew | בז נודד |
| Hungarian | Vándorsólyom |
| Icelandic | Förufálki |
| Indonesian | Alap-alap kawah |
| Italian | Falco pellegrino |
| Japanese | ハヤブサ |
| Korean | 매 |
| Latvian | Lielais piekūns |
| Lithuanian | Sakalas keleivis |
| Malayalam | കായൽപ്പുള്ള് |
| Marathi | शाहीन |
| Mongolian | Эгэл шонхор |
| Norwegian | vandrefalk |
| Persian | شاهین بحری |
| Polish | sokół wędrowny |
| Portuguese (Angola) | Falcão-peregrino |
| Portuguese (Brazil) | falcão-peregrino |
| Portuguese (Portugal) | Falcão-peregrino |
| Romanian | Șoim călător |
| Russian | Сапсан |
| Serbian | Sivi soko |
| Slovak | sokol sťahovavý |
| Slovenian | Sokol selec |
| Spanish | Halcón Peregrino |
| Spanish (Argentina) | Halcón Peregrino |
| Spanish (Chile) | Halcón peregrino |
| Spanish (Costa Rica) | Halcón Peregrino |
| Spanish (Cuba) | Halcón peregrino |
| Spanish (Dominican Republic) | Halcón Peregrino |
| Spanish (Ecuador) | Halcón Peregrino |
| Spanish (Honduras) | Halcón Peregrino |
| Spanish (Mexico) | Halcón Peregrino |
| Spanish (Panama) | Halcón Peregrino |
| Spanish (Paraguay) | Halcón peregrino |
| Spanish (Peru) | Halcón Peregrino |
| Spanish (Puerto Rico) | Falcón Peregrino |
| Spanish (Spain) | Halcón peregrino |
| Spanish (Uruguay) | Halcón Peregrino |
| Spanish (Venezuela) | Halcón Peregrino |
| Swedish | pilgrimsfalk |
| Thai | เหยี่ยวเพเรกริน |
| Turkish | Gökdoğan |
| Ukrainian | Сапсан |
Falco peregrinus Tunstall, 1771
PROTONYM:
Falco Peregrinus
Tunstall, 1771. Ornithologia Britannica: seu avium omnium Britannicarum tam terrestrium, quam aquaticarum catalogus (etc.), p.1.
TYPE LOCALITY:
Northamptonshire, England.)
SOURCE:
Avibase, 2023
Definitions
- FALCO
- falco
- peregrinus
The Key to Scientific Names
Legend Overview
UPPERCASE: current genus
Uppercase first letter: generic synonym
● and ● See: generic homonyms
lowercase: species and subspecies
●: early names, variants, misspellings
‡: extinct
†: type species
Gr.: ancient Greek
L.: Latin
<: derived from
syn: synonym of
/: separates historical and modern geographic names
ex: based on
TL: type locality
OD: original diagnosis (genus) or original description (species)
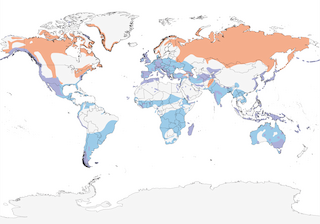
- Year-round
- Migration
- Breeding
- Non-Breeding
Distribution of the Peregrine Falcon





































