Osprey Pandion haliaetus Scientific name definitions
- LC Least Concern
- Names (68)
- Subspecies (4)
Richard O. Bierregaard, Alan F. Poole, Mark S. Martell, Peter Pyle, and Michael A. Patten
Version: 1.0 — Published March 4, 2020
Sign in to see your badges
Species names in all available languages
| Language | Common name |
|---|---|
| Afrikaans | Visvalk |
| Albanian | Shqiponja peshkngrënëse |
| Arabic | عقاب نساري |
| Armenian | Ջրարծիվ |
| Asturian | ñguila pescadora |
| Azerbaijani | Çay qaraquşu |
| Basque | Arrano arrantzalea |
| Bulgarian | Орел рибар |
| Catalan | àguila pescadora |
| Chinese | 魚鷹 |
| Chinese (SIM) | 鹗 |
| Croatian | bukoč |
| Czech | orlovec říční |
| Danish | Fiskeørn |
| Dutch | Visarend |
| English | Osprey |
| English (United States) | Osprey |
| Faroese | Fiskiørn |
| Finnish | sääksi |
| French | Balbuzard pêcheur |
| French (France) | Balbuzard pêcheur |
| Galician | Aguia pescadora |
| German | Fischadler |
| Greek | Ψαραετός |
| Haitian Creole (Haiti) | Malfini lanmè |
| Hebrew | שלך |
| Hungarian | Halászsas |
| Icelandic | Gjóður |
| Indonesian | Elang tiram |
| Italian | Falco pescatore |
| Japanese | ミサゴ |
| Korean | 물수리 |
| Latvian | Zivju ērglis |
| Lithuanian | Žuvininkas |
| Malayalam | താലിപ്പരുന്ത് |
| Marathi | कैकर |
| Mongolian | Загасч явлаг |
| Norwegian | fiskeørn |
| Persian | عقاب ماهیگیر |
| Polish | rybołów |
| Portuguese (Angola) | Águia-pesqueira |
| Portuguese (Brazil) | águia-pescadora |
| Portuguese (Portugal) | Águia-pesqueira |
| Romanian | Uligan pescar |
| Russian | Скопа |
| Serbian | Ribar |
| Slovak | kršiak rybár |
| Slovenian | Ribji orel |
| Spanish | Águila Pescadora |
| Spanish (Argentina) | Aguila Pescadora |
| Spanish (Chile) | Águila pescadora |
| Spanish (Costa Rica) | Águila Pescadora |
| Spanish (Cuba) | Guincho |
| Spanish (Dominican Republic) | Guincho |
| Spanish (Ecuador) | Águila Pescadora |
| Spanish (Honduras) | Águila Pescadora |
| Spanish (Mexico) | Águila Pescadora |
| Spanish (Panama) | Águila Pescadora |
| Spanish (Paraguay) | Águila pescadora |
| Spanish (Peru) | Aguila Pescadora |
| Spanish (Puerto Rico) | Águila Pescadora |
| Spanish (Spain) | Águila pescadora |
| Spanish (Uruguay) | Águila Pescadora |
| Spanish (Venezuela) | Águila Pescadora |
| Swedish | fiskgjuse/australisk fiskgjuse |
| Thai | เหยี่ยวออสเปร |
| Turkish | Balık Kartalı |
| Ukrainian | Скопа західна |
Pandion haliaetus (Linnaeus, 1758)
PROTONYM:
Falco Halioetus
Linnaeus, 1758. Systema Naturæ per Regna Tria Naturæ, Secundum Classes, Ordines, Genera, Species, cum Characteribus, Differentiis, Synonymis, Locis. Tomus I. Editio decima, reformata 1, p.91.
TYPE LOCALITY:
Europe; restricted to Sweden by Linnaeus, 1761 Fauna Svecica, ed. 2, p. 22.
SOURCE:
Avibase, 2023
Definitions
- PANDION
- pandion
- haliaetus
The Key to Scientific Names
Legend Overview
UPPERCASE: current genus
Uppercase first letter: generic synonym
● and ● See: generic homonyms
lowercase: species and subspecies
●: early names, variants, misspellings
‡: extinct
†: type species
Gr.: ancient Greek
L.: Latin
<: derived from
syn: synonym of
/: separates historical and modern geographic names
ex: based on
TL: type locality
OD: original diagnosis (genus) or original description (species)
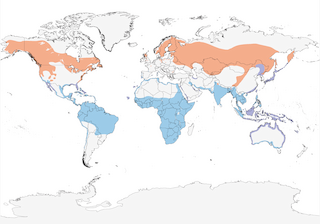
- Year-round
- Migration
- Breeding
- Non-Breeding
Distribution of the Osprey
































