Northern Pintail Anas acuta Scientific name definitions
- LC Least Concern
- Names (67)
- Monotypic
Robert G. Clark, Joseph P. Fleskes, Karla L. Guyn, David A. Haukos, Jane E. Austin, and Michael R. Miller
Version: 1.0 — Published March 4, 2020
Sign in to see your badges
Species names in all available languages
| Language | Common name |
|---|---|
| Afrikaans | Pylsterteend |
| Albanian | Rosa bishtgjelë |
| Arabic | بلبول شمالي |
| Armenian | Նետապոչ բադ |
| Asturian | Corñu rabudu |
| Azerbaijani | Bizquyruq ördək |
| Basque | Ahate buztanluzea |
| Bulgarian | Шилоопашата патица |
| Catalan | ànec cuallarg |
| Chinese | 尖尾鴨 |
| Chinese (SIM) | 针尾鸭 |
| Croatian | patka lastarka |
| Czech | ostralka štíhlá |
| Danish | Spidsand |
| Dutch | Pijlstaart |
| English | Northern Pintail |
| English (HAW) | Koloa mapu - Northern Pintail |
| English (United States) | Northern Pintail |
| Faroese | Snælduont |
| Finnish | jouhisorsa |
| French | Canard pilet |
| French (France) | Canard pilet |
| Galician | Pato albelo |
| German | Spießente |
| Greek | Ψαλίδα |
| Gujarati | સિંગપર |
| Haitian Creole (Haiti) | Kanna pilè |
| Hebrew | ברווז חד-זנב |
| Hungarian | Nyílfarkú réce |
| Icelandic | Grafönd |
| Indonesian | Itik utara |
| Italian | Codone |
| Japanese | オナガガモ |
| Kannada | ಸೂಜಿಬಾಲದ ಬಾತು |
| Korean | 고방오리 |
| Latvian | Garkaklis |
| Lithuanian | Smailiauodegė antis |
| Malayalam | വാലൻ എരണ്ട |
| Marathi | तलवार बदक |
| Mongolian | Шовтгор алаг нугас |
| Norwegian | stjertand |
| Odia | ଗହିର ଲଞ୍ଜା |
| Persian | فیلوش |
| Polish | rożeniec |
| Portuguese (Brazil) | arrabio |
| Portuguese (Portugal) | Arrábio |
| Punjabi (India) | ਸੀਂਖਪਰ |
| Romanian | Rață sulițar |
| Russian | Шилохвость |
| Serbian | Šiljkan |
| Slovak | kačica ostrochvostá |
| Slovenian | Dolgorepa raca |
| Spanish | Ánade Rabudo |
| Spanish (Costa Rica) | Pato Rabudo |
| Spanish (Cuba) | Pato pescuecilargo |
| Spanish (Dominican Republic) | Pato Guineo |
| Spanish (Ecuador) | Ánade Norteño |
| Spanish (Honduras) | Yaguasa Coluda |
| Spanish (Mexico) | Pato Golondrino |
| Spanish (Panama) | Ánade Rabudo |
| Spanish (Puerto Rico) | Pato Pescuecilargo |
| Spanish (Spain) | Ánade rabudo |
| Spanish (Venezuela) | Pato Rabudo |
| Swedish | stjärtand |
| Thai | เป็ดหางแหลม |
| Turkish | Kılkuyruk |
| Ukrainian | Шилохвіст північний |
Anas acuta Linnaeus, 1758
PROTONYM:
Anas acuta
Linnaeus, 1758. Systema Naturæ per Regna Tria Naturæ, Secundum Classes, Ordines, Genera, Species, cum Characteribus, Differentiis, Synonymis, Locis. Tomus I. Editio decima, reformata 1, p.126.
TYPE LOCALITY:
Europe; restricted to Sweden by Linnaeus, 1761, Fauna Svecica, ed. 2, p. 44.
SOURCE:
Avibase, 2023
Definitions
- ANAS
- acuta
The Key to Scientific Names
Legend Overview
UPPERCASE: current genus
Uppercase first letter: generic synonym
● and ● See: generic homonyms
lowercase: species and subspecies
●: early names, variants, misspellings
‡: extinct
†: type species
Gr.: ancient Greek
L.: Latin
<: derived from
syn: synonym of
/: separates historical and modern geographic names
ex: based on
TL: type locality
OD: original diagnosis (genus) or original description (species)
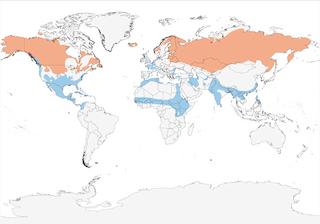
- Year-round
- Migration
- Breeding
- Non-Breeding
Distribution of the Northern Pintail