Eastern Bluebird Sialia sialis Scientific name definitions
- LC Least Concern
- Names (24)
- Subspecies (7)
Patricia A. Gowaty and Jonathan H. Plissner
Version: 1.0 — Published March 4, 2020
Text last updated February 9, 2015
Text last updated February 9, 2015
Sign in to see your badges
Species names in all available languages
| Language | Common name |
|---|---|
| Catalan | siàlia oriental |
| Croatian | riđoprsa modrorepka |
| Czech | salašník modrý |
| Dutch | Roodkeelsialia |
| English | Eastern Bluebird |
| English (United States) | Eastern Bluebird |
| French | Merlebleu de l'Est |
| French (France) | Merlebleu de l'Est |
| German | Rotkehl-Hüttensänger |
| Icelandic | Spegilskotta |
| Japanese | ルリツグミ |
| Norwegian | østblåfugl |
| Polish | błękitnik rudogardły |
| Russian | Восточная сиалия |
| Serbian | Istočna plavica |
| Slovak | salašník modrochrbtý |
| Spanish | Azulejo Oriental |
| Spanish (Cuba) | Azulejo pechirrojo |
| Spanish (Honduras) | Zorzalito Azul |
| Spanish (Mexico) | Azulejo Garganta Canela |
| Spanish (Spain) | Azulejo oriental |
| Swedish | östsialia |
| Turkish | Doğulu Mavi Ardıç |
| Ukrainian | Блакитник східний |
Sialia sialis (Linnaeus, 1758)
PROTONYM:
Motacilla Sialis
Linnaeus, 1758. Systema Naturæ per Regna Tria Naturæ, Secundum Classes, Ordines, Genera, Species, cum Characteribus, Differentiis, Synonymis, Locis. Tomus I. Editio decima, reformata 1, p.187.
TYPE LOCALITY:
in Bermudis & America calidiore [= South Carolina].
SOURCE:
Avibase, 2023
Definitions
- SIALIA
- sialis
- Sialis
The Key to Scientific Names
Legend Overview
UPPERCASE: current genus
Uppercase first letter: generic synonym
● and ● See: generic homonyms
lowercase: species and subspecies
●: early names, variants, misspellings
‡: extinct
†: type species
Gr.: ancient Greek
L.: Latin
<: derived from
syn: synonym of
/: separates historical and modern geographic names
ex: based on
TL: type locality
OD: original diagnosis (genus) or original description (species)
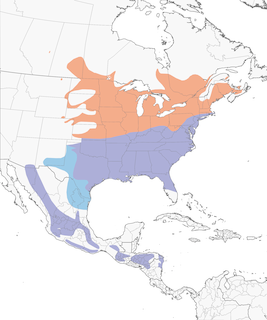
- Year-round
- Migration
- Breeding
- Non-Breeding
Distribution of the Eastern Bluebird




























