Double-crested Cormorant Nannopterum auritum Scientific name definitions
- LC Least Concern
- Names (37)
- Subspecies (5)
Brian S. Dorr, Jeremy J. Hatch, and D. V. Weseloh
Version: 1.1 — Published August 18, 2021
Sign in to see your badges
Species names in all available languages
| Language | Common name |
|---|---|
| Catalan | corb marí orellut |
| Croatian | žutogrli vranac |
| Czech | kormorán ušatý |
| Danish | Øreskarv |
| Dutch | Geoorde Aalscholver |
| English | Double-crested Cormorant |
| English (United States) | Double-crested Cormorant |
| Finnish | amerikanmerimetso |
| French | Cormoran à aigrettes |
| French (France) | Cormoran à aigrettes |
| Galician | Corvo mariño de orellas |
| German | Ohrenscharbe |
| Greek | Αμερικάνικος Κορμοράνος |
| Haitian Creole (Haiti) | Kòmoran lanmè |
| Hebrew | קורמורן אמריקני |
| Hungarian | Füles kárókatona |
| Icelandic | Skerjaskarfur |
| Japanese | ミミヒメウ |
| Lithuanian | Ausuotasis kormoranas |
| Norwegian | totoppskarv |
| Polish | kormoran rogaty |
| Portuguese (Portugal) | Corvo-marinho-d'orelhas |
| Romanian | Cormoran urechiat |
| Russian | Ушастый баклан |
| Serbian | Žutogrli vranac |
| Slovak | kormorán ušatý |
| Slovenian | Zlatogrli kormoran |
| Spanish | Cormorán Orejudo |
| Spanish (Cuba) | Corúa de mar |
| Spanish (Dominican Republic) | Corúa |
| Spanish (Honduras) | Cormorán Crestado |
| Spanish (Mexico) | Cormorán Orejón |
| Spanish (Puerto Rico) | Cormorán Crestado |
| Spanish (Spain) | Cormorán orejudo |
| Swedish | öronskarv |
| Turkish | Kulaklı Karabatak |
| Ukrainian | Баклан вухатий |
Nannopterum auritum (Lesson, 1831)
PROTONYM:
Carbo auritus
Lesson, 1831. Traité d'Ornithologie, ou Tableau Méthodique des ordres, sous-ordres, familles, tribus, genres, sous-genres et races d'oiseaux livr.8, p.605.
TYPE LOCALITY:
New Zealand; error, North America.
SOURCE:
Avibase, 2023
Definitions
- NANNOPTERUM
- auritum / auritus
The Key to Scientific Names
Legend Overview
UPPERCASE: current genus
Uppercase first letter: generic synonym
● and ● See: generic homonyms
lowercase: species and subspecies
●: early names, variants, misspellings
‡: extinct
†: type species
Gr.: ancient Greek
L.: Latin
<: derived from
syn: synonym of
/: separates historical and modern geographic names
ex: based on
TL: type locality
OD: original diagnosis (genus) or original description (species)
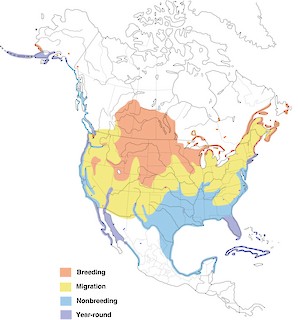
- Year-round
- Migration
- Breeding
- Non-Breeding
Figure 1. Distribution of the Double-crested Cormorant.
This species also winters in Bermuda in small numbers, and winters locally south to the dashed line. See text for details.



























