Cliff Swallow Petrochelidon pyrrhonota Scientific name definitions
- LC Least Concern
- Names (45)
- Subspecies (4)
Charles R. Brown, Mary B. Brown, Peter Pyle, and Michael A. Patten
Version: 1.0 — Published March 4, 2020
Text last updated February 6, 2017
Text last updated February 6, 2017
Sign in to see your badges
Species names in all available languages
| Language | Common name |
|---|---|
| Bulgarian | Американска брегова лястовица |
| Catalan | oreneta dels cingles |
| Czech | vlaštovka pestrá |
| Danish | Stensvale |
| Dutch | Amerikaanse Klifzwaluw |
| English | Cliff Swallow |
| English (United States) | Cliff Swallow |
| French | Hirondelle à front blanc |
| French (France) | Hirondelle à front blanc |
| German | Fahlstirnschwalbe |
| Greek | Αμερικανικό Γκρεμοχελίδονο |
| Haitian Creole (Haiti) | Irondèl fwon blanch |
| Hebrew | סנונית אמריקנית |
| Hungarian | Sziklafecske |
| Icelandic | Klettasvala |
| Japanese | サンショクツバメ |
| Lithuanian | Baltakaktė klifinė kregždė |
| Norwegian | mursvale |
| Polish | jaskółka rdzawoszyja |
| Portuguese (Brazil) | andorinha-de-dorso-acanelado |
| Portuguese (Portugal) | Andorinha-de-testa-branca |
| Romanian | Rândunică de stâncă |
| Russian | Белолобая ласточка |
| Serbian | Američka lasta litičarka |
| Slovak | lastovička bralová |
| Slovenian | Pečinska lastovka |
| Spanish | Golondrina Risquera |
| Spanish (Argentina) | Golondrina Rabadilla Canela |
| Spanish (Chile) | Golondrina grande |
| Spanish (Costa Rica) | Golondrina Risquera |
| Spanish (Cuba) | Golondrina de cuevas americana |
| Spanish (Dominican Republic) | Golondrina de Farallón |
| Spanish (Ecuador) | Golondrina de Riscos |
| Spanish (Honduras) | Golondrina Risquera |
| Spanish (Mexico) | Golondrina Risquera |
| Spanish (Panama) | Golondrina Risquera |
| Spanish (Paraguay) | Golondrina rabadilla canela |
| Spanish (Peru) | Golondrina Risquera |
| Spanish (Puerto Rico) | Golondrina de Peñasco |
| Spanish (Spain) | Golondrina risquera |
| Spanish (Uruguay) | Golondrina Rabadilla Canela |
| Spanish (Venezuela) | Golondrina Risquera |
| Swedish | stensvala |
| Turkish | Amerika Kırlangıcı |
| Ukrainian | Ясківка білолоба |
Petrochelidon pyrrhonota (Vieillot, 1817)
PROTONYM:
Hirundo pyrrhonota
Vieillot, 1817. Nouveau dictionnaire d'histoire naturelle, appliquée aux arts, à l'agriculture, à l'économie rurale et domestique, à la médecine, etc. Nouvelle édition 14, p.519.
TYPE LOCALITY:
Paraguay.
SOURCE:
Avibase, 2023
Definitions
- PETROCHELIDON
- pyrrhonota / pyrrhonotha / pyrrhonotus
The Key to Scientific Names
Legend Overview
UPPERCASE: current genus
Uppercase first letter: generic synonym
● and ● See: generic homonyms
lowercase: species and subspecies
●: early names, variants, misspellings
‡: extinct
†: type species
Gr.: ancient Greek
L.: Latin
<: derived from
syn: synonym of
/: separates historical and modern geographic names
ex: based on
TL: type locality
OD: original diagnosis (genus) or original description (species)
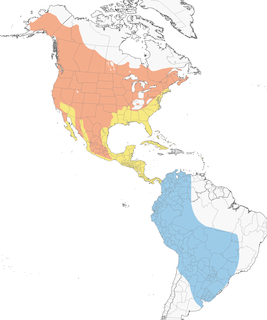
- Year-round
- Migration
- Breeding
- Non-Breeding
Distribution of the Cliff Swallow





























