American Golden-Plover Pluvialis dominica Scientific name definitions
- LC Least Concern
- Names (58)
- Monotypic
Oscar W. Johnson, Peter G. Connors, and Peter Pyle
Version: 1.1 — Published April 15, 2021
Revision Notes
Version: 1.1 — Published April 15, 2021
Revision Notes
Sign in to see your badges
Species names in all available languages
| Language | Common name |
|---|---|
| Afrikaans | Amerikaanse Goue Strandkiewiet |
| Arabic | زقزاق ذهبي امريكي |
| Asturian | Pilordu dorñu americanu |
| Basque | Urre-txirri amerikarra |
| Bulgarian | Доминиканска булка |
| Catalan | daurada americana |
| Chinese (SIM) | 美洲金鸻 |
| Croatian | američki zlatar |
| Czech | kulík hnědokřídlý |
| Danish | Amerikansk Hjejle |
| Dutch | Amerikaanse Goudplevier |
| English | American Golden-Plover |
| English (UK) | American Golden Plover |
| English (United Arab Emirates) | American Golden Plover |
| English (United States) | American Golden-Plover |
| Finnish | amerikankurmitsa |
| French | Pluvier bronzé |
| French (France) | Pluvier bronzé |
| Galician | Píllara dourada americana |
| German | Prärie-Goldregenpfeifer |
| Greek | Αμερικανικό Βροχοπούλι |
| Haitian Creole (Haiti) | Plivye savann |
| Hebrew | חופזי אמריקני |
| Hungarian | Amerikai pettyeslile |
| Icelandic | Gulllóa |
| Italian | Piviere americano |
| Japanese | アメリカムナグロ |
| Korean | 미국검은가슴물떼새 |
| Lithuanian | Amerikinis sėjikas |
| Malayalam | അമേരിക്കൻ പൊൻമണൽക്കോഴി |
| Norwegian | kanadalo |
| Polish | siewka szara |
| Portuguese (Brazil) | batuiruçu |
| Portuguese (Portugal) | Batuiruçu |
| Romanian | Ploier auriu american |
| Russian | Американская ржанка |
| Serbian | Američki zlatni vivak |
| Slovak | kulík hnedokrídly |
| Slovenian | Ameriška zlata prosenka |
| Spanish | Chorlito Dorado Americano |
| Spanish (Argentina) | Chorlo Pampa |
| Spanish (Chile) | Chorlo dorado |
| Spanish (Costa Rica) | Chorlito Dorado Menor |
| Spanish (Cuba) | Pluvial dorado |
| Spanish (Dominican Republic) | Chorlo Americano |
| Spanish (Ecuador) | Chorlo Dorado Americano |
| Spanish (Honduras) | Chorlo Dorado Americano |
| Spanish (Mexico) | Chorlo Dorado Americano |
| Spanish (Panama) | Chorlo Dorado Americano |
| Spanish (Paraguay) | Chorlo dorado |
| Spanish (Peru) | Chorlo Dorado Americano |
| Spanish (Puerto Rico) | Chorlito Dorado |
| Spanish (Spain) | Chorlito dorado americano |
| Spanish (Uruguay) | Chorlo Dorado |
| Spanish (Venezuela) | Playero Dorado |
| Swedish | amerikansk tundrapipare |
| Turkish | Amerika Altın Yağmurcunu |
| Ukrainian | Сивка американська |
Revision Notes
In this partial revision, Oscar W. Johnson updated the Effects of Human Activity section.
Pluvialis dominica (Müller, 1776)
PROTONYM:
Charadrius Dominicius
Müller, 1776. Des Ritters Carl von Linné ... vollständigen Natursystems Supplements- und Register-Band über alle sechs Theile oder Classen des Thierreichs, p.116.
TYPE LOCALITY:
Hispaniola.
SOURCE:
Avibase, 2023
Definitions
- PLUVIALIS
- pluvialis
- dominica
The Key to Scientific Names
Legend Overview
UPPERCASE: current genus
Uppercase first letter: generic synonym
● and ● See: generic homonyms
lowercase: species and subspecies
●: early names, variants, misspellings
‡: extinct
†: type species
Gr.: ancient Greek
L.: Latin
<: derived from
syn: synonym of
/: separates historical and modern geographic names
ex: based on
TL: type locality
OD: original diagnosis (genus) or original description (species)
Account navigation Account navigation
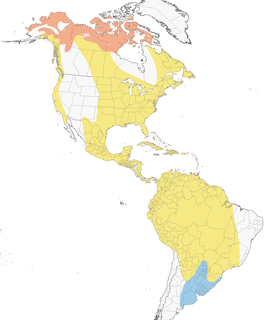
- Year-round
- Migration
- Breeding
- Non-Breeding
Distribution of the American Golden-Plover











