American Redstart Setophaga ruticilla Scientific name definitions
- LC Least Concern
- Names (43)
- Monotypic
Thomas W. Sherry, Richard T. Holmes, Peter Pyle, and Michael A. Patten
Version: 1.0 — Published March 4, 2020
Text last updated December 2, 2016
Text last updated December 2, 2016
Sign in to see your badges
Species names in all available languages
| Language | Common name |
|---|---|
| Catalan | bosquerola cua-roja |
| Croatian | crna sjeničica |
| Czech | lesňáček lejsčí |
| Danish | Amerikansk Ildstjert |
| Dutch | Amerikaanse Roodstaart |
| English | American Redstart |
| English (United States) | American Redstart |
| French | Paruline flamboyante |
| French (France) | Paruline flamboyante |
| German | Rotschwanz-Waldsänger |
| Greek | Αμερικανικός Φοινίκουρος |
| Haitian Creole (Haiti) | Ti Tchit dife |
| Hebrew | סבכון חכלילי |
| Hungarian | Legyezőfarkú lombposzáta |
| Icelandic | Húmskríkja |
| Japanese | ハゴロモムシクイ |
| Lithuanian | Gelsvauodegis krūminukas |
| Norwegian | rødstjertparula |
| Polish | lasówka szkarłatna |
| Portuguese (Brazil) | mariquita-de-rabo-vermelho |
| Portuguese (Portugal) | Mariquita-de-rabo-vermelho |
| Romanian | Codroș american |
| Russian | Горихвостковая древесница |
| Serbian | Američka crvenrepka |
| Slovak | horárik čierny |
| Slovenian | Muharski peničar |
| Spanish | Candelita Norteña |
| Spanish (Argentina) | Arañero Boreal |
| Spanish (Chile) | Candelita americana |
| Spanish (Costa Rica) | Candelita Norteña |
| Spanish (Cuba) | Candelita |
| Spanish (Dominican Republic) | Bijirita |
| Spanish (Ecuador) | Candelita Norteña |
| Spanish (Honduras) | Chipe Negro y Anaranjado |
| Spanish (Mexico) | Pavito Migratorio |
| Spanish (Panama) | Candelita Norteña |
| Spanish (Peru) | Candelita Americana |
| Spanish (Puerto Rico) | Candelita |
| Spanish (Spain) | Candelita norteña |
| Spanish (Venezuela) | Candelita Migratoria |
| Swedish | rödstjärtad skogssångare |
| Turkish | Kızıl Kuyruklu Ötleğen |
| Ukrainian | Пісняр горихвістковий |
Setophaga ruticilla (Linnaeus, 1758)
PROTONYM:
Motacilla ruticilla
Linnaeus, 1758. Systema Naturæ per Regna Tria Naturæ, Secundum Classes, Ordines, Genera, Species, cum Characteribus, Differentiis, Synonymis, Locis. Tomus I. Editio decima, reformata 1, p.186.
TYPE LOCALITY:
America ; restricted to Virginia by Amer. Ornith. Union, 1910, Check-list North Amer. Birds, ed. 3, p. 326
SOURCE:
Avibase, 2023
Definitions
- SETOPHAGA
- ruticilla
- Ruticilla
The Key to Scientific Names
Legend Overview
UPPERCASE: current genus
Uppercase first letter: generic synonym
● and ● See: generic homonyms
lowercase: species and subspecies
●: early names, variants, misspellings
‡: extinct
†: type species
Gr.: ancient Greek
L.: Latin
<: derived from
syn: synonym of
/: separates historical and modern geographic names
ex: based on
TL: type locality
OD: original diagnosis (genus) or original description (species)
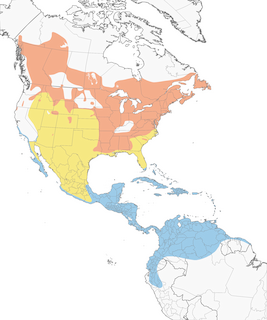
- Year-round
- Migration
- Breeding
- Non-Breeding
Distribution of the American Redstart



























