Acorn Woodpecker Melanerpes formicivorus Scientific name definitions
- LC Least Concern
- Names (25)
- Subspecies (7)
Walter D. Koenig, Eric L. Walters, Peter B. Stacey, Mark T. Stanback, and Ronald L. Mumme
Version: 1.0 — Published March 4, 2020
Sign in to see your badges
Species names in all available languages
| Language | Common name |
|---|---|
| Bulgarian | Жълъдов кълвач |
| Catalan | picot menjaglans |
| Czech | datel sběrač |
| Dutch | Eikelspecht |
| English | Acorn Woodpecker |
| English (United States) | Acorn Woodpecker |
| French | Pic glandivore |
| French (France) | Pic glandivore |
| German | Eichelspecht |
| Icelandic | Eikarspæta |
| Japanese | ドングリキツツキ |
| Norwegian | eikespett |
| Polish | dzięciur żołędziowy |
| Russian | Желудёвый дятел |
| Serbian | Žirov detlić |
| Slovak | tesárik dubový |
| Spanish | Carpintero Bellotero |
| Spanish (Costa Rica) | Carpintero Careto |
| Spanish (Honduras) | Carpintero de Robledal |
| Spanish (Mexico) | Carpintero Bellotero |
| Spanish (Panama) | Carpintero Careto |
| Spanish (Spain) | Carpintero bellotero |
| Swedish | samlarspett |
| Turkish | Palamut Ağaçkakanı |
| Ukrainian | Гіла чорновола |
Melanerpes formicivorus (Swainson, 1827)
PROTONYM:
Picus formicivorus
Swainson, 1827. The Philosophical Magazine, New Series n.s., 1, p.439.
TYPE LOCALITY:
Temiscaltepec, Mexico.
SOURCE:
Avibase, 2023
Definitions
- MELANERPES
- formicivorus
- Formicivorus
The Key to Scientific Names
Legend Overview
UPPERCASE: current genus
Uppercase first letter: generic synonym
● and ● See: generic homonyms
lowercase: species and subspecies
●: early names, variants, misspellings
‡: extinct
†: type species
Gr.: ancient Greek
L.: Latin
<: derived from
syn: synonym of
/: separates historical and modern geographic names
ex: based on
TL: type locality
OD: original diagnosis (genus) or original description (species)
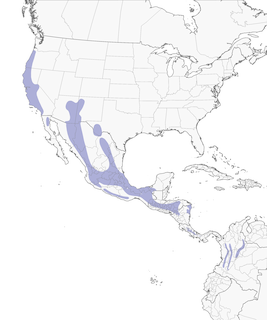
- Year-round
- Migration
- Breeding
- Non-Breeding
Distribution of the Acorn Woodpecker


























